Standardize Randomization for Reproducibility
The Reproducibility Crisis in Preclinical Research
Multiple large-scale analyses have highlighted a significant reproducibility crisis in preclinical research, where a majority of findings cannot be consistently replicated. For instance, Amgen reported that only 11% of 53 landmark cancer studies could be replicated [Begley & Ellis, Nature 2012], while Bayer found that only 20–25% of 67 published studies were reproducible [Prinz et al., Nat Rev Drug Discov 2011].
A major contributor to this crisis is the lack of methodological rigor in experimental design, particularly concerning how animals are allocated to treatment groups. When randomization is performed manually or without proper documentation, results become impossible to verify, audit, or reproduce, undermining scientific integrity.
What Scientific Guidelines Recommend
The most widely adopted guidelines for animal research consistently emphasize that randomization must be documented, standardized, and transparently reported to ensure scientific rigor and enhance reproducibility:
- ARRIVE 2.0 Guidelines [Percie du Sert et al., PLoS Biol 2020] — These guidelines require explicit reporting of the type of randomization used, the precise method employed to generate the allocation sequence, and the unit of randomization. Randomization is listed as an Essential 10 item for publication.
- PREPARE Guidelines [Smith et al., Lab Anim 2018] — Emphasizes that comprehensive experimental planning, including the randomization strategy, must be formalized before the study commences to ensure the highest standards of quality and reproducibility.
- NIH Rigor and Reproducibility [Collins & Tabak, Nature 2014] — Mandates that grant applicants clearly describe their plans for randomization, blinding, and statistical analysis as integral components of their experimental design, promoting robust and reproducible research.
Why Standardizing Randomization Matters
Implementing a consistent, well-documented randomization method across all your preclinical experiments offers profound benefits, fostering greater confidence in your research outcomes:
- Enhanced Traceability — Every randomization performed with Randmice generates a detailed report, including the algorithm used, number of iterations, convergence analysis, and the full group assignment. This provides an indisputable audit trail, allowing you to trace back exactly how each animal was allocated.
- Improved Reproducibility — A standardized and automated randomization protocol eliminates potential operator bias and ensures that different team members or laboratories can consistently follow the exact same procedure, thereby enhancing the reproducibility of results.
- Simplified Compliance — Journals, funding agencies, and ethical committees increasingly require a comprehensive description of the randomization methodology. Having a structured, readily available report significantly streamlines manuscript preparation, grant applications, and regulatory audits.
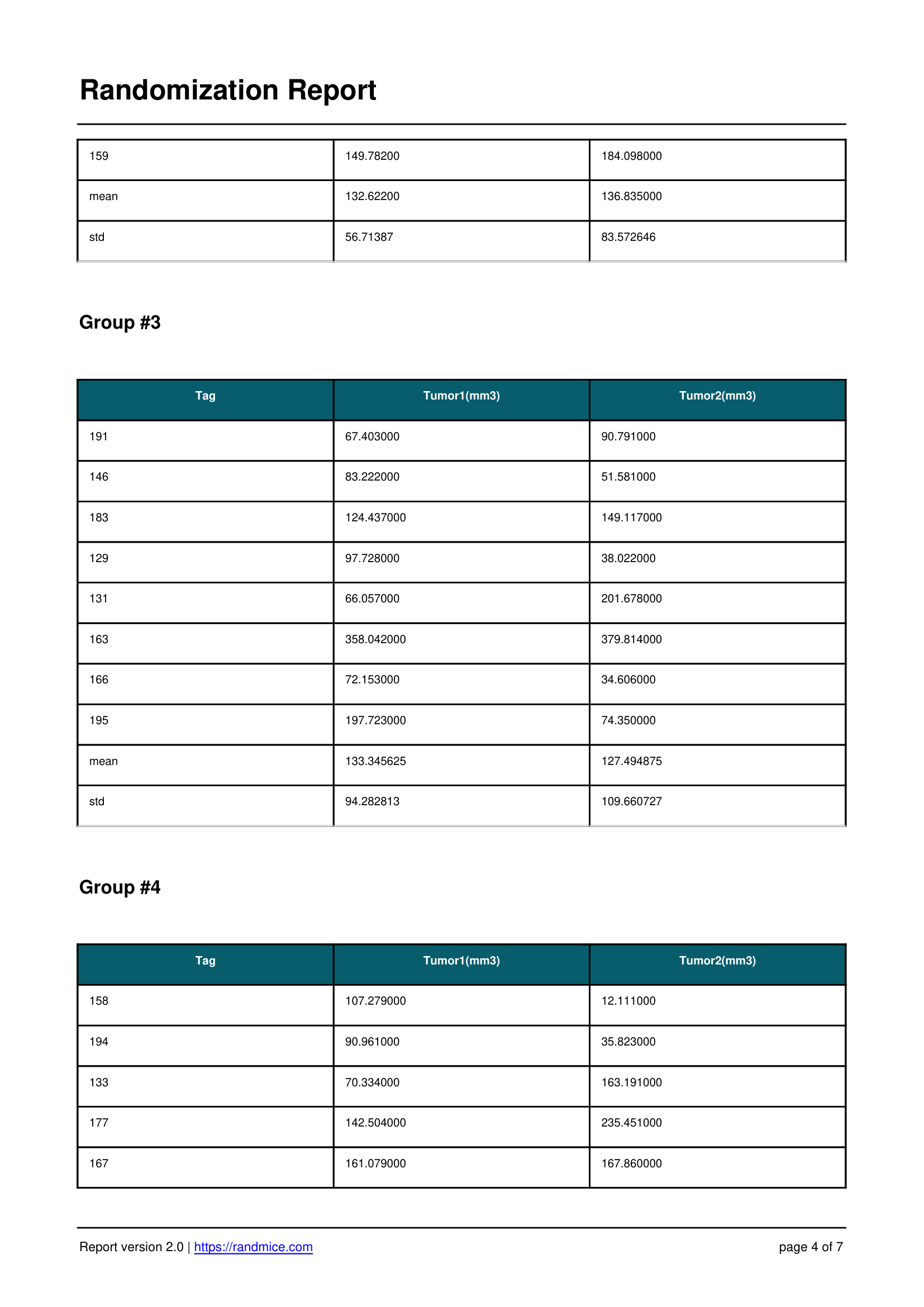
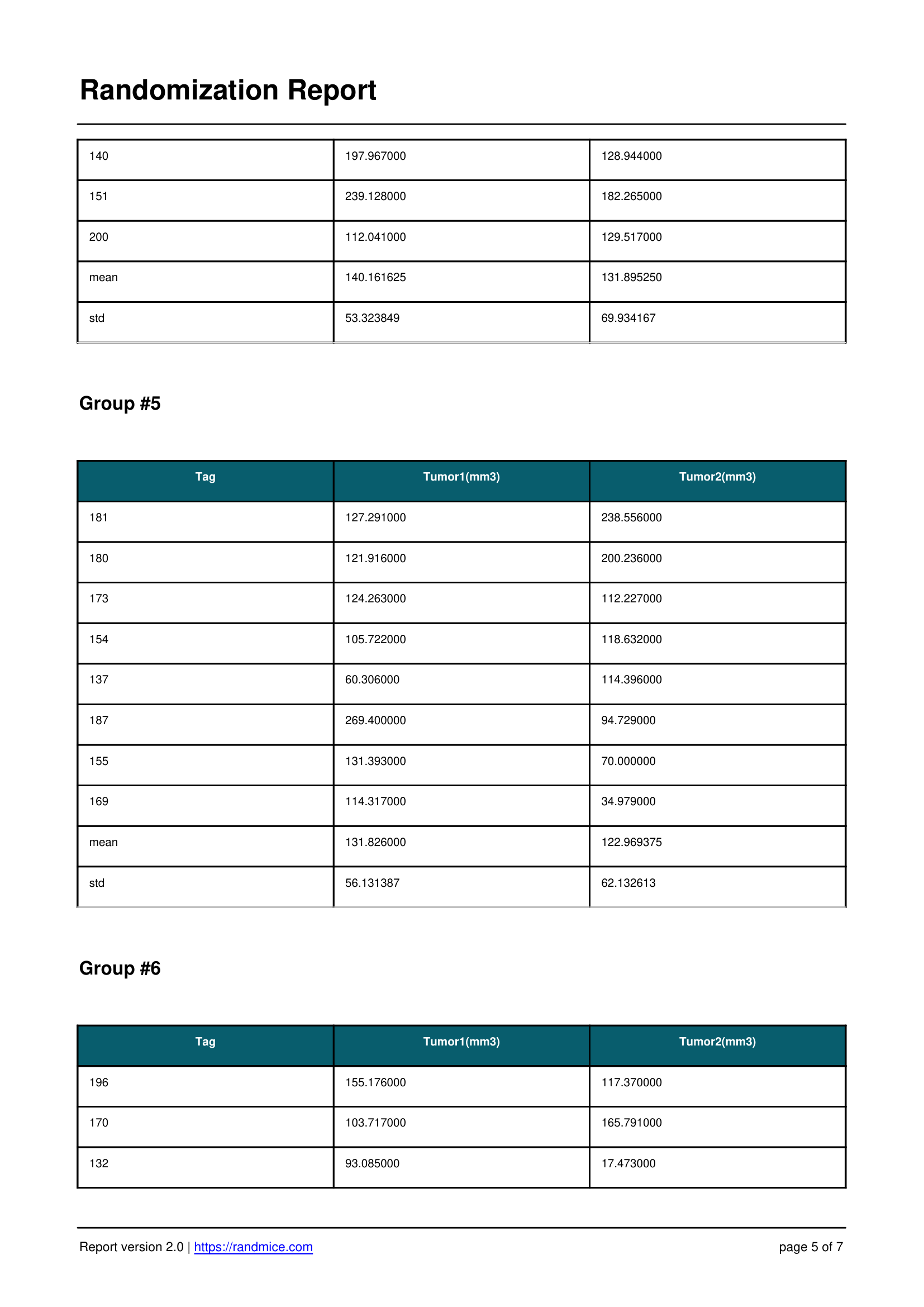
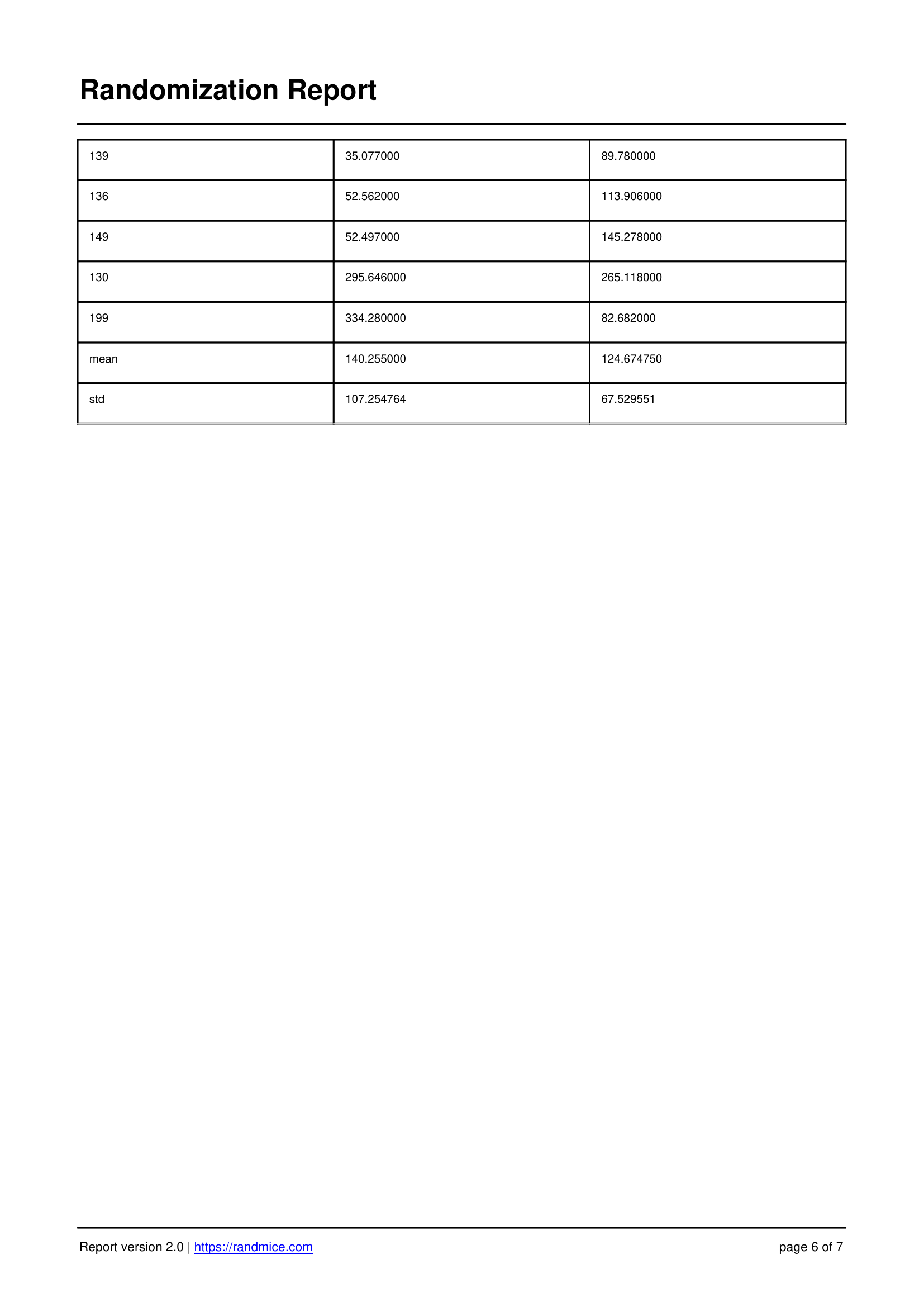
A Detailed Report for Every Experiment
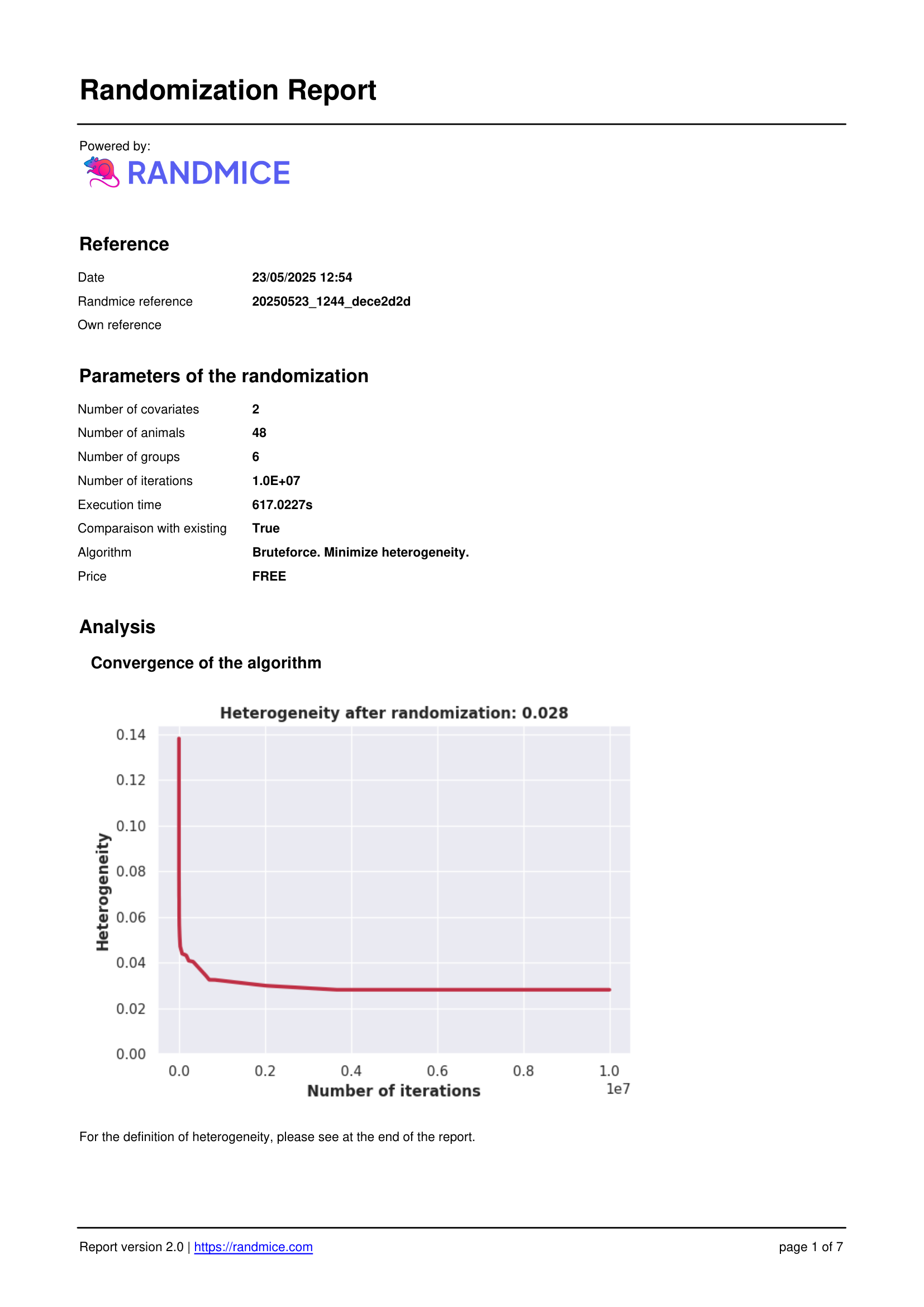
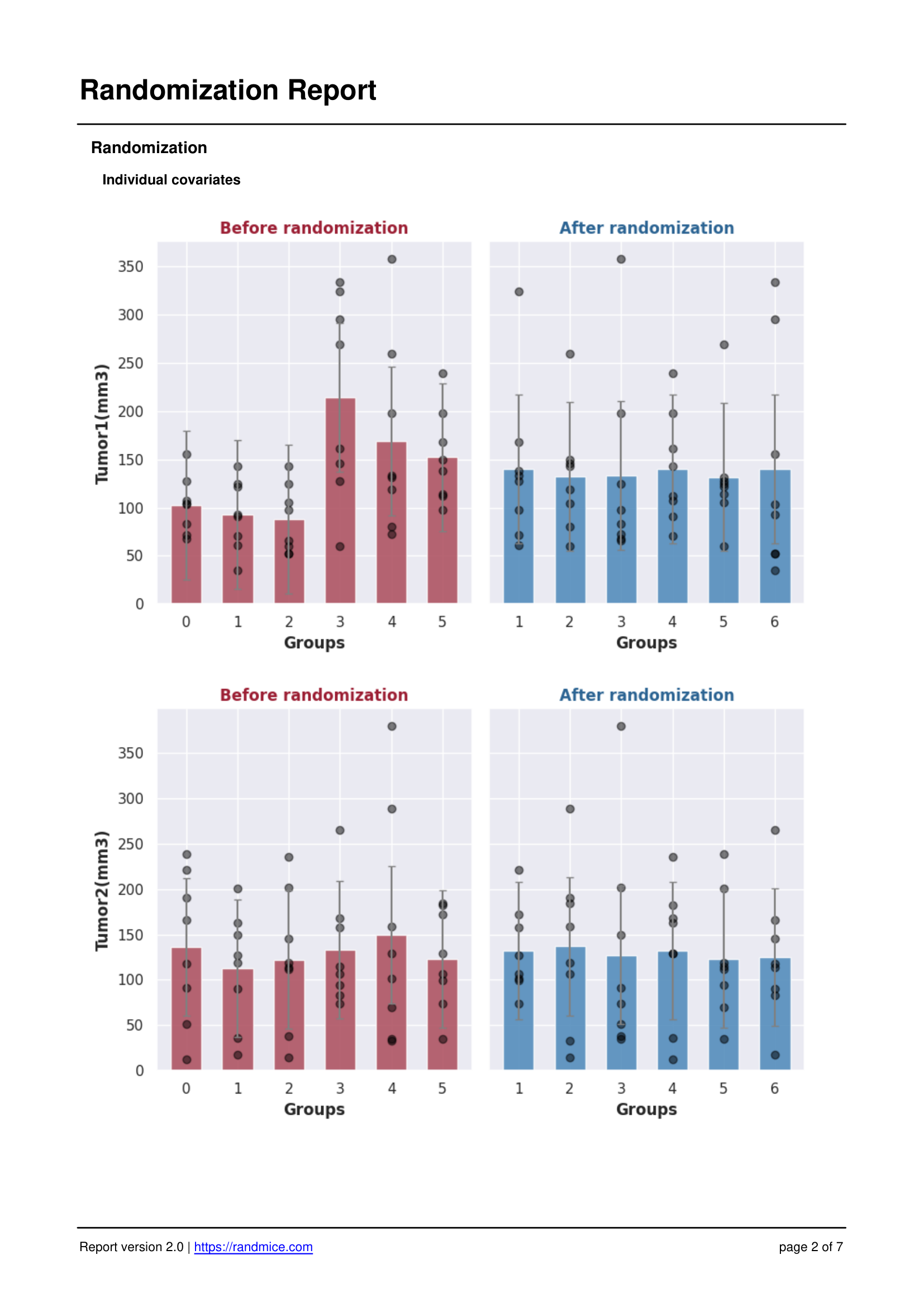
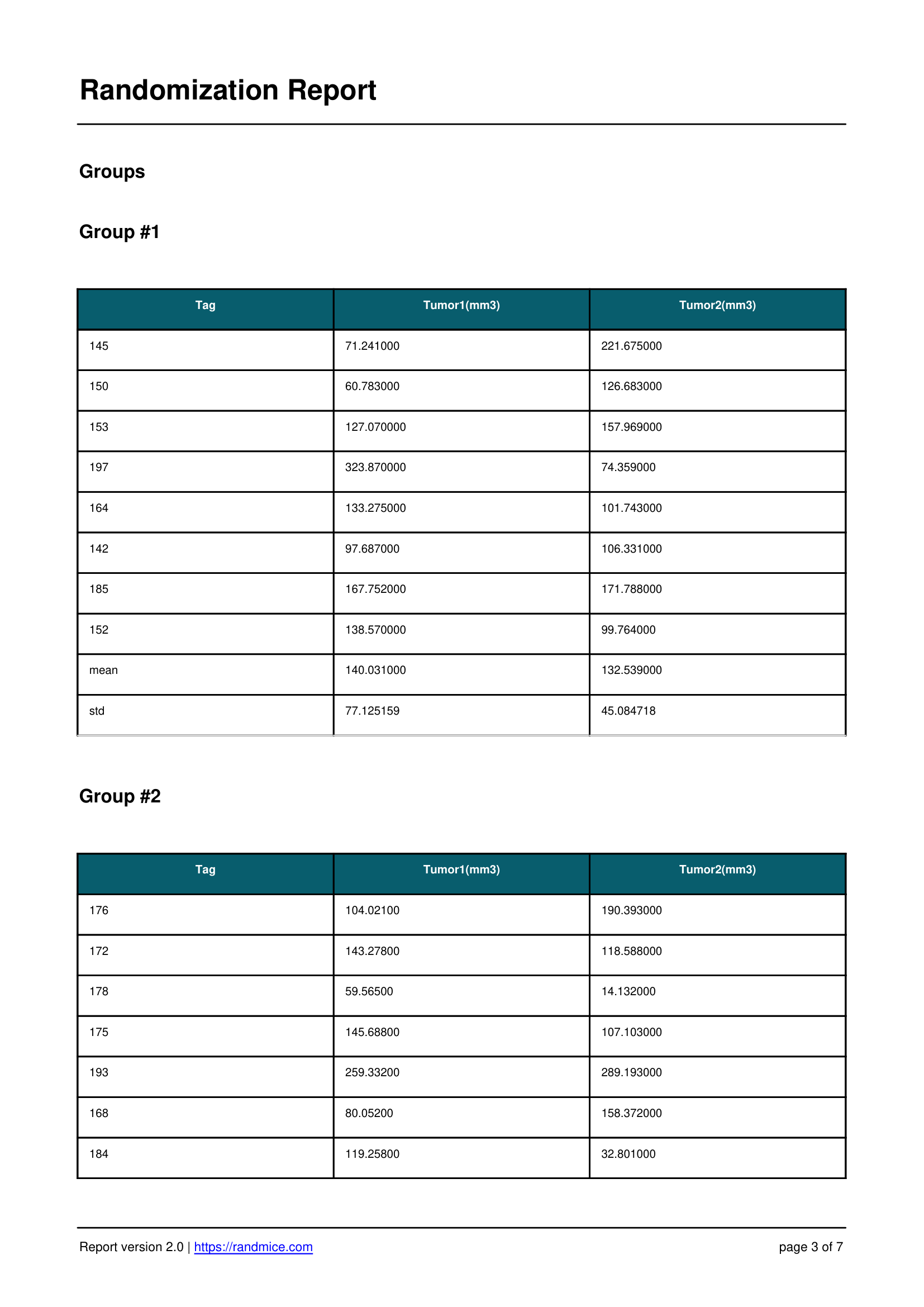
Each randomization performed with Randmice automatically generates a comprehensive PDF report that includes: the date of the run, a unique reference ID, all experimental parameters (number of covariates, animals, groups, iterations), the convergence curve, individual animal assignments, detailed group statistics, and a ready-to-use material & methods paragraph for easy inclusion in publications.

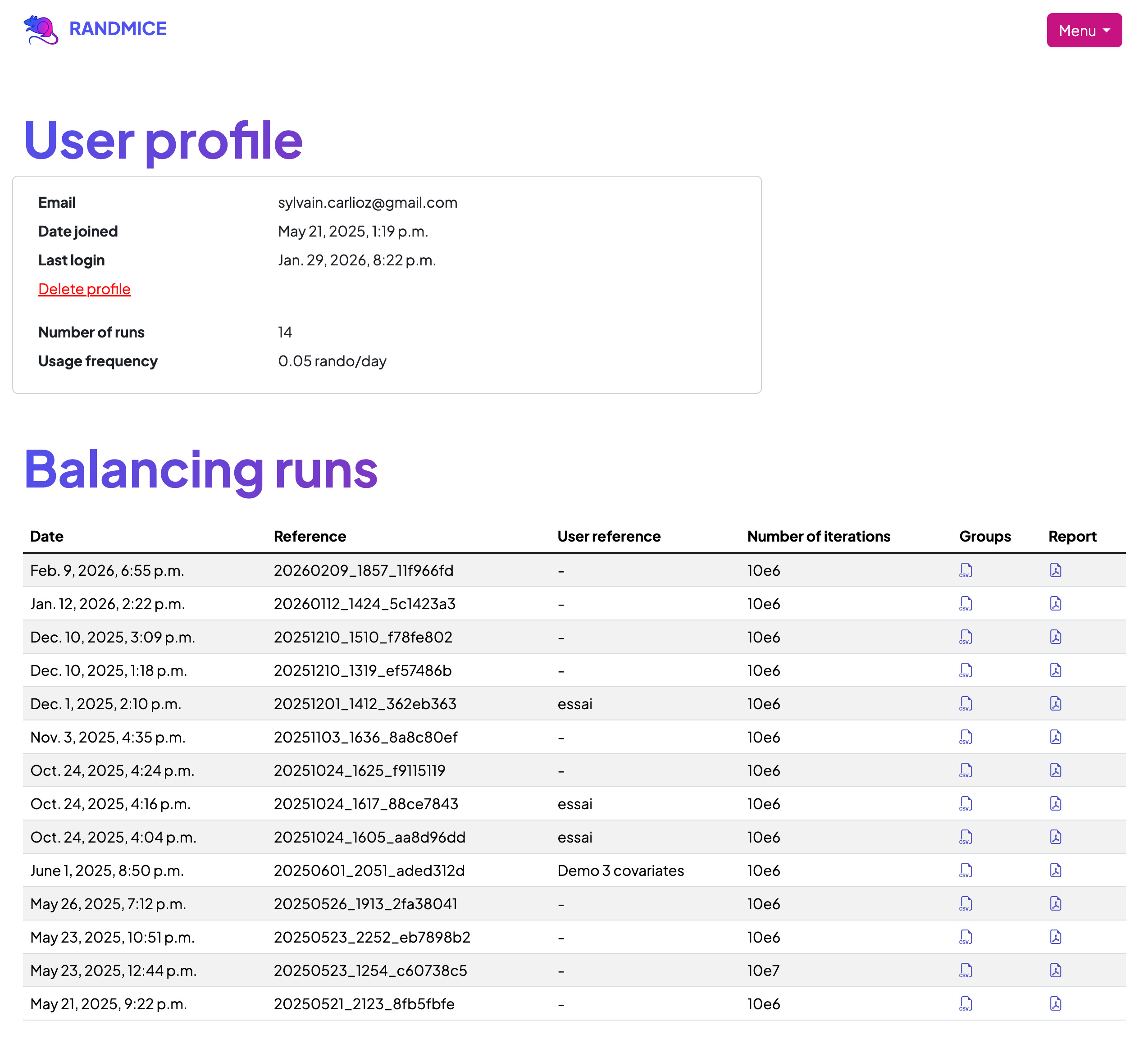
Centralize All Your Reports in One Place
With a free Randmice account, all your randomization reports are securely stored in a personal dashboard. You gain immediate access to sort, view, and download any past report—including CSV data files and PDF reports—at any time.
Eliminate the hassle of scattered files across emails, USB drives, or shared folders. Randmice provides one centralized location, one standardized format, and full traceability for all your randomization data, significantly improving data management and accessibility.